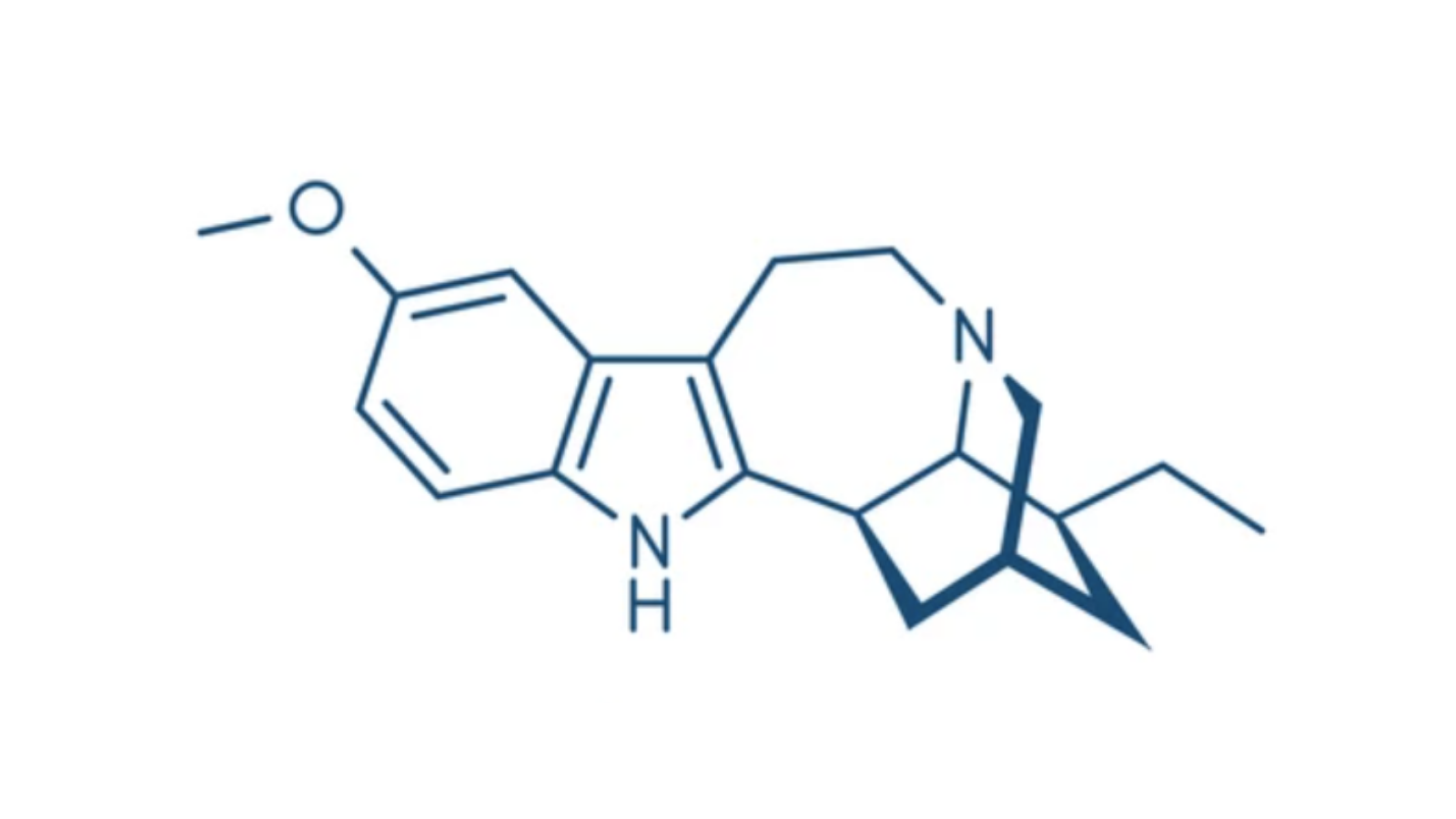
Psychedelic profiles ibogaine
Ibogaine is a psychoactive compound found in several plants, most notably in the roots of the iboga tree, and has been used medicinally and ceremoniously in West Africa for centuries.
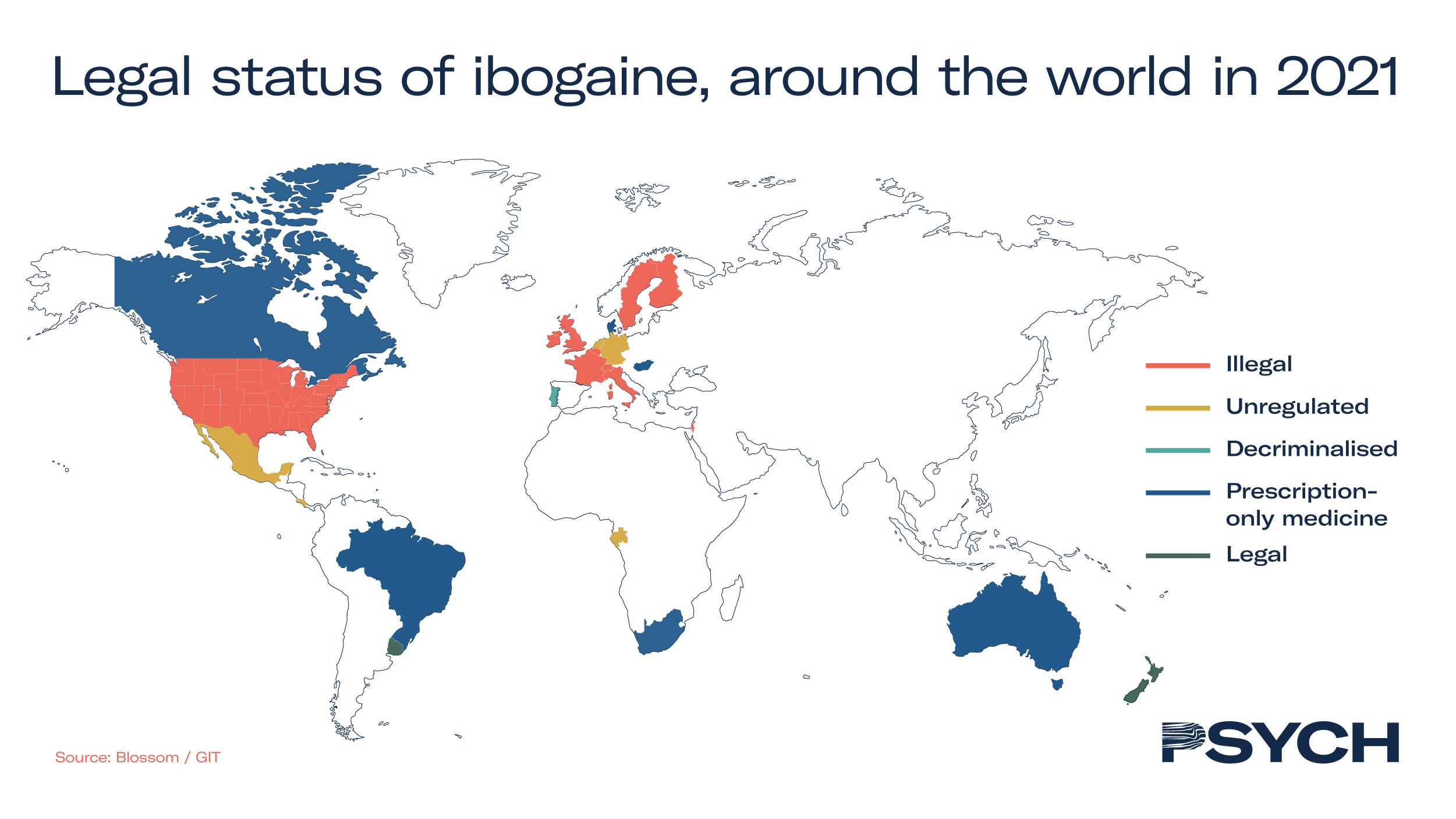
Although ibogaine is not listed as a scheduled substance by the United Nations, possession of it is strictly regulated or prohibited in several Western countries – restricting its adoption. The map below illustrates the regulatory status of ibogaine around the world.
 The entheogen has an established history in the treatment of substance use disorders, particularly in South America where its use remains unregulated. Ibogaine has the potential to decrease substance misuse, particularly opioid use disorder, which has become an epidemic across North America.
The entheogen has an established history in the treatment of substance use disorders, particularly in South America where its use remains unregulated. Ibogaine has the potential to decrease substance misuse, particularly opioid use disorder, which has become an epidemic across North America.
Clinical trials
Two clinical trials are currently underway to investigate ibogaine’s medical utility, in the treatment of methadone detoxification and alcoholism. Observational studies funded by MAPS, at independent treatment centres in Mexico and New Zealand, found ibogaine has the potential to decrease a patient’s misuse of opiates and symptoms of withdrawal after a single dose.
However, ibogaine consumption can have an adverse impact on the cardiovascular system, resulting in over 20 recorded fatalities in the past 30 years. A Dutch study at Radboud University found nearly half of the patients showed a delay in ventricular repolarisation, the time it takes for the heart muscle to recharge between beats, of 0.45 seconds following administration.
Due to its relatively low safety profile, particularly in comparison to other psychedelic medicines, several drug developers are researching ibogaine analogues with mitigated risks.
DemeRx, in partnership with atai Life Sciences, is developing both ibogaine and noribogaine, ibogaine’s principal psychoactive metabolite, as treatments for opioid dependence. The latter has a greater affinity for opioid receptors than ibogaine and in March 2021 DemeRx received approval from the MHRA to conduct clinical trials in the UK.
First synthesised in a laboratory in 1996, 18-Methoxycoronaridine (18-MC) is an ibogaine derivative developed to reduce the substance’s impact on heartbeat regularity. In 2014, Savant HWP filed an IND application with the FDA for 18-MC; however, the regulatory review was stalled until MindMed acquired the company in 2019.
In January 2022, MindMed completed its Phase 1 clinical trial with 18-MC in the treatment of opioid withdrawal and opioid use disorder, with topline results expected later this year.
Robert Barrow, Chief Executive Officer and Director of MindMed, commented, ‘The growing opioid crisis claims over 75,000 lives each year…While ibogaine has been used and studied as a treatment for opioid addiction, its efficacy, while promising, has been overshadowed by significant safety concerns. Our proprietary molecule, 18-MC, has indicated an encouraging safety profile and preclinical efficacy data.’
Delix Therapeutics has developed a completely non-hallucinogenic analogue, tabernanthalog. In rodents the compound has been shown to have anti-addictive properties and to promote neural plasticity. However, it remains to be seen if the non-hallucinogenic nature of tabernanthalog undermines its efficacy in humans.
Commercial opportunity
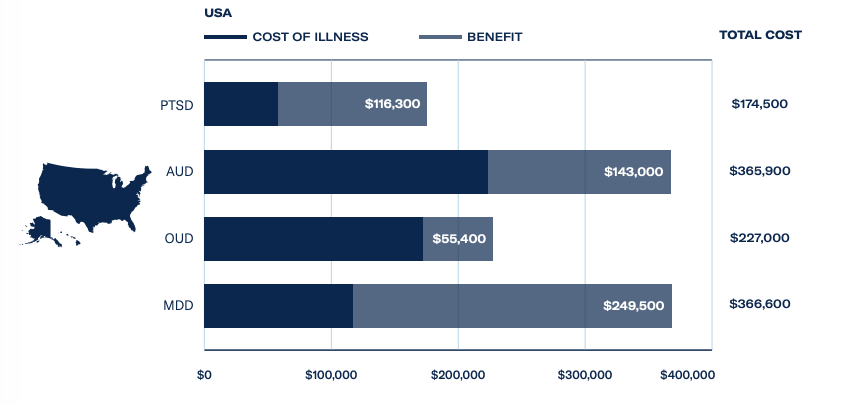
There has been a backlash against the negative side effects of long-term use and addiction to opioids, with over 75,000 deaths attributed to opioid overdose in the US alone. Opioid use disorder costs the US economy US$227 billion a year and ibogaine treatments are rarely covered by health insurance providers. When paid for privately, a course of treatment can be priced between US$5,000 and US$8,000 – presenting lucrative opportunities for treatment providers.
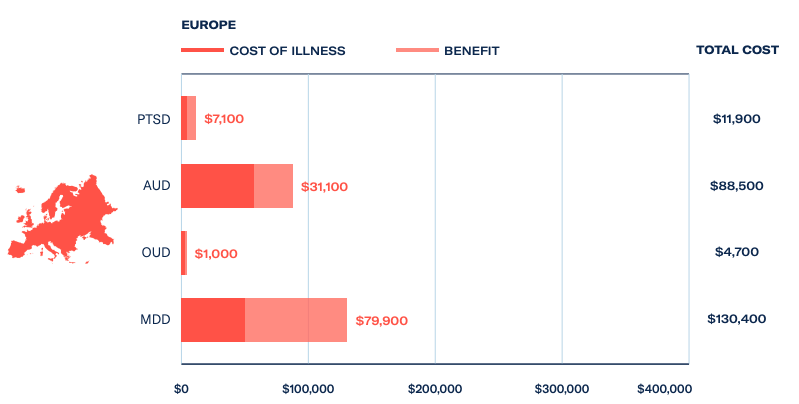
In Europe, where pharmaceutical opioids are more rigorously controlled, opioid use disorder still costs the economy US$4.7 billion a year. In response, a number of ibogaine treatment centres have been established in favourable jurisdictions where the medicine is either available on prescription or unregulated.
 For further intelligence on the psychedelic healthcare industry, and to make informed investment decisions, download The Psychedelics as Medicine Report: Third Edition, powered by Blossom.
For further intelligence on the psychedelic healthcare industry, and to make informed investment decisions, download The Psychedelics as Medicine Report: Third Edition, powered by Blossom.


