On Tuesday, 29 November, PSYCH released the fourth edition of The Psychedelics as Medicine Report.
The report, expanded to include data on adult-use frameworks, contains market valuations and contributions from business leaders, researchers and regulators, empowering investors to make informed decisions.
It revealed the industry is currently worth US$650 million, and is expected to exceed US$3 billion by 2026. The next three years will be pivotal in the adoption of psychedelic healthcare, with MDMA and psilocybin-assisted therapies expected to be regulated on both sides of the Atlantic.
The Psychedelics as Medicine Report
To navigate the rapidly changing landscape, The Psychedelics as Medicine Report provides a holistic overview of the interventions poised for market approval, and a balanced perspective of the related socioeconomic opportunities.
MAPS’ Founder Rick Doblin commented: ‘There is a big change that’s happened with the rise of for-profit companies. The fact that PSYCH and PSYCH industry reports exist is very exciting.’
The comprehensive account of the industry contains market projections for ketamine, MDMA and psilocybin-assisted therapies, with significant inflection points expected in the next three years. As psychedelic medicines progress to the final stages of drug development, attention is turning to commercialisation and the expected patient journey.
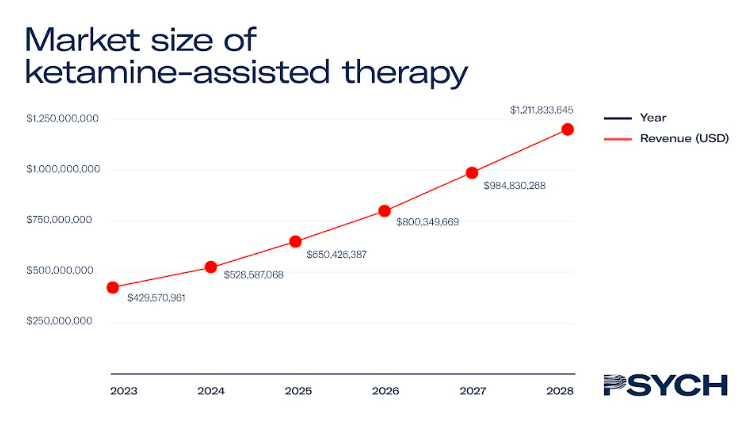
Ketamine-assisted therapy
Ketamine-assisted therapy typically costs US$600 per session, and revenue from ketamine treatments is expected to exceed US$230 million by 2023. As the only psychedelic medicine currently approved for medical use, ketamine-assisted therapy will account for the majority of the market until 2027.
Speaking to PSYCH for the report, Awakn Life Sciences’ Head of Psychedelic Medicine, Dr Ben Sessa, said: ‘I have no doubt that in the future psychedelic medicine will be available for free on the NHS for a few reasons – it is safe, it’s effective and it is cheap.
‘It may sound expensive, when a patient has to pay £6,000 themselves… but it actually makes total economic sense. I’m quite convinced that the NHS will put these medicines into public healthcare because psychedelic-assisted therapies make sense economically.’
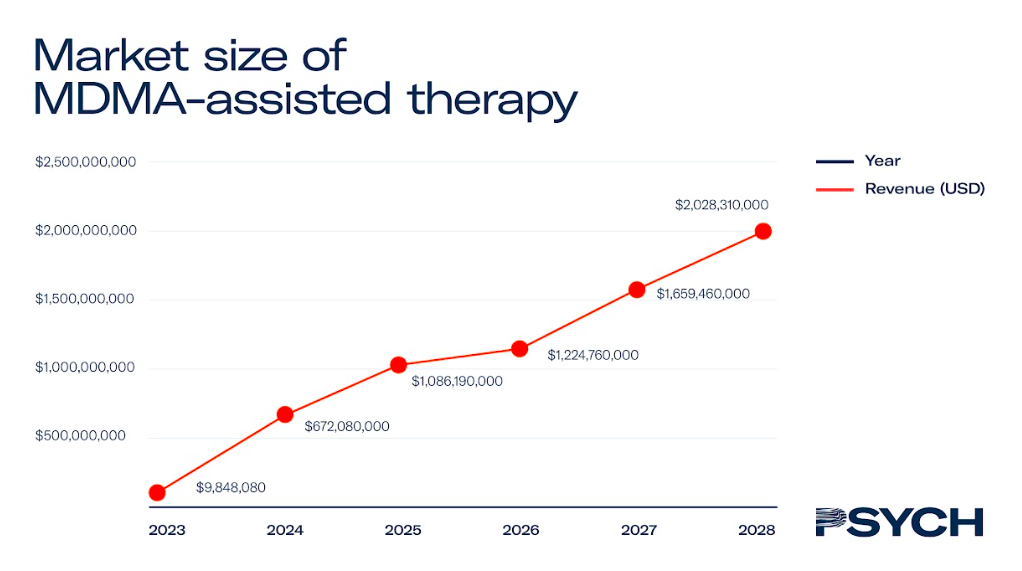
MDMA-assisted therapy
MAPS’ MDMA-assisted therapy for PTSD is the only psychedelic therapy in Phase III clinical trials, with regulatory approval expected in 2024.
In North America, the treatment is expected to cost US$7,543 – with patients undertaking two courses of therapy a year until they are in remission. Assuming the price of MDMA-assisted therapy stays consistent, and that there is a linear growth rate in the number of trained therapists, the market for MDMA-assisted therapy could surpass US$1.5 billion by 2028.
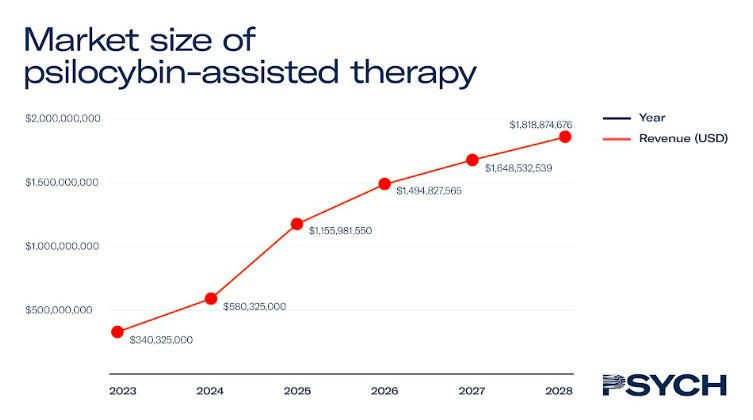
Psilocybin-assisted therapy
After ketamine, psilocybin is the most studied psychedelic medicine, with over 70 clinical trials conducted on its therapeutic efficacy.
Last year, UK-based COMPASS Pathways shared data from its Phase II trial to combat treatment-resistant depression, with its proprietary psilocybin formulation, COMP360. If similar results can be replicated in Phase III studies, COMP360 therapy could be available to patients with treatment-resistant depression by 2025.
The report estimates that if enough therapists are trained to administer the psychedelic medicine, the value of the North American market could reach US$1.3 billion by 2028.
The Psychedelics as Medicine Report: Fourth Edition was sponsored by Awakn Life Sciences, Cybin Inc., Psyence Group, Negev Capital and Psych Capital.
Market data and insights have been compiled from across the industry for a holistic and impartial perspective on the commercial potential of psychedelic healthcare. This is substantiated by modelling created in collaboration with drug developers, healthcare providers and financial analysts.
To learn more about the potential of psychedelic healthcare, please download a complimentary copy of the report.