
This week, the Multidisciplinary Association for Psychedelic Studies (MAPS) released findings from a groundbreaking Phase 3 clinical trial that suggest MDMA-assisted therapy will be an effective treatment for severe, chronic post-traumatic stress disorder (PTSD).
‘In our first Phase 3 study, roughly one third of the people had PTSD for 20 years or more, and yet two thirds of them no longer have PTSD at the end of the study,’ MAPS founder Rick Doblin told PSYCH.
The randomised blinded trial treated 90 patients with severe, chronic PTSD; participants were treated with either MDMA or a placebo, with identical talk therapy, over three sessions.
Forty-six participants received MDMA therapy, and forty-four participants received therapy with placebo. Researchers assessed changes in participants’ functional impairment in work/school, social and family life after 18 weeks.
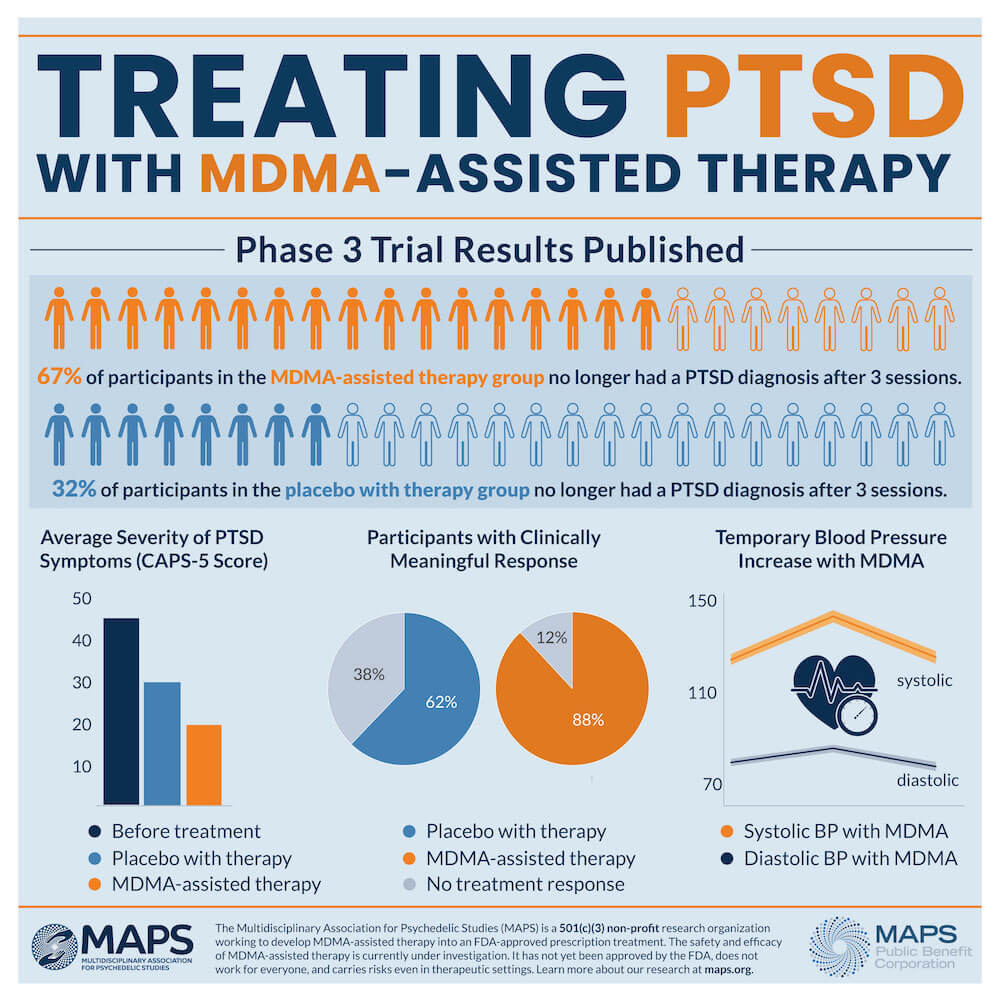
Among the participants in the MDMA-assisted therapy group, 67% no longer qualified for PTSD diagnosis after three MDMA-assisted therapy sessions and 88% of participants experienced a clinically significant reduction in symptoms; while in the placebo group, 32% no longer qualified for PTSD diagnosis at the two-month follow-up and 60% experienced a clinically significant reduction in symptoms.
The statistically significant results are a boon to the non-profit’s long-term goals of introducing structured practitioner-assisted MDMA therapy to patients on a broad scale. MAPS is hopeful that these results will facilitate FDA approval in 2023 for the breakthrough-designated therapy.
‘The more that neuroscience research can show neuroplasticity and new neural connections, the more people can then give credence. If people – meaning regulators, key opinion leaders – can give credence to these changes, not only can they happen, but they can be durable. I think we’re going to really see more of that developing,’ Doblin said.

The California-based non-profit, founded in 1986, is sponsoring the most advanced psychedelic-therapy research in the world. It received breakthrough designation from the FDA in 2017, aligning on special protocol assessment for its Phase 3 trials.
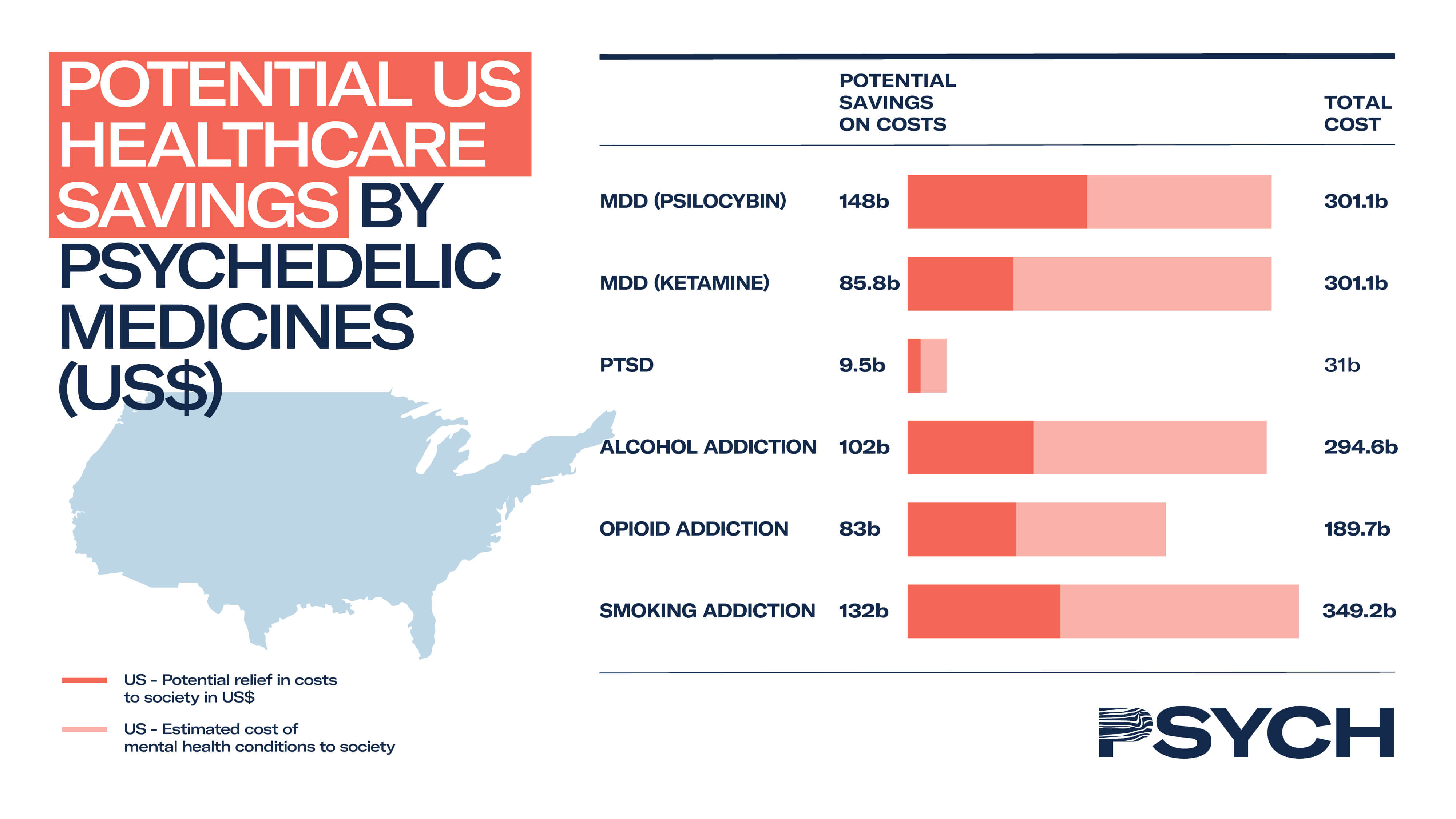
Proprietary PSYCH data suggests psychedelic medicine for PTSD could result in healthcare savings of US$9.5 billion in the United States, and US$2.9 billion in Europe.
Since PSYCH’s data was gathered, MAPS has calculated in precise detail how MDMA-assisted therapy could provide superior treatment to existing therapies at significantly reduced costs.
‘We’re already having discussions with various health insurance companies about it. I think that’s going to be key. The good thing for us in Europe is that the national healthcare is there to pay for treatment, but they also pay for disability payments. And so for PTSD, when you consider severe PTSD is very disabling depression as well, the economic cost-effectiveness study makes more sense,’ Doblin told PSYCH.
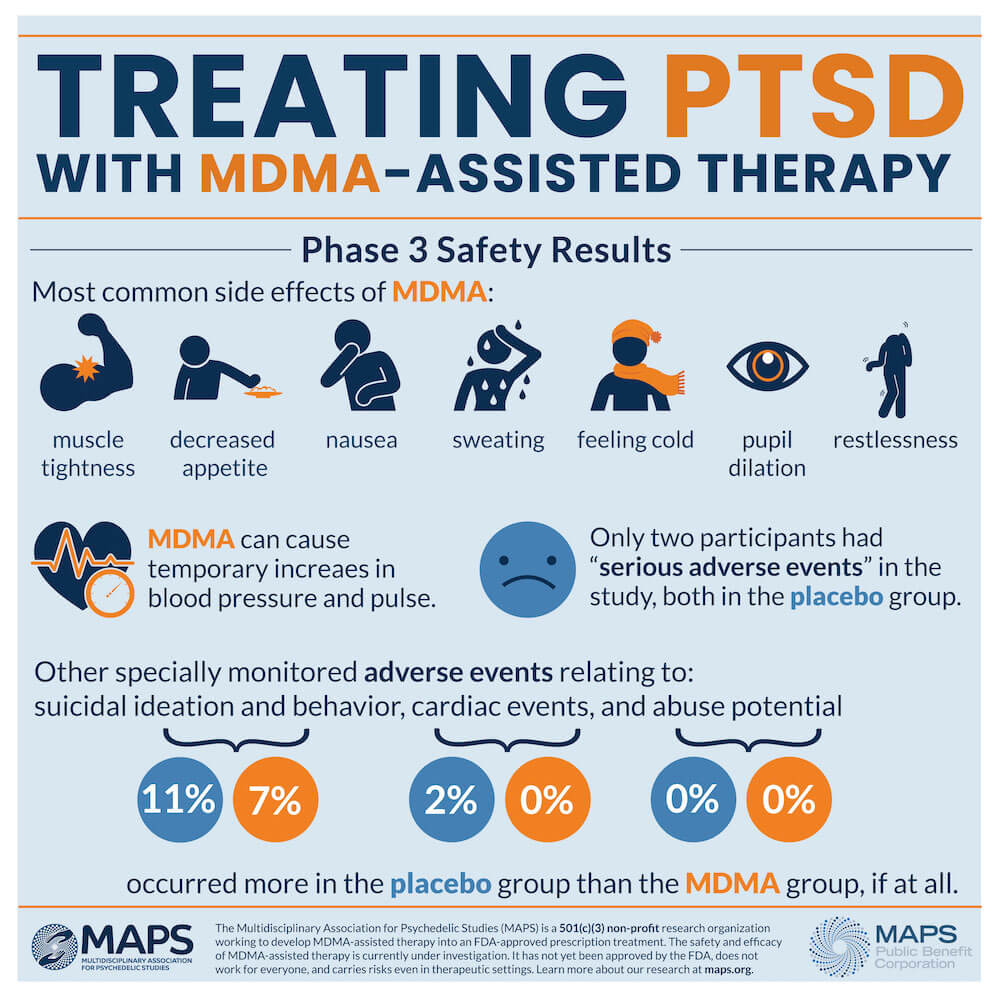
Through the course of the study, investigators observed no serious safety or tolerability issues in the MDMA group. Temporary increases in blood pressure and pulse were observed, and adverse reactions, including muscle tightness, decreased appetite, nausea, sweating and feeling cold, were transient.
Currently listed as a Schedule I drug, MDMA presently is defined as having ‘no medical benefit’ and is not currently accessible as a potential treatment for PTSD or for other conditions except as administered in clinical trials.
‘What’s going to primarily change culture is stories of healing, from a broad cultural base. Also, we need the key opinion leaders for that – we need the science, we need the data. So when the FDA says yes to MDMA-assisted therapy for PTSD, assuming that happens roughly two years from now, that’s the first key proof of principle, that psychedelic-assisted therapy can make it through the regulatory system. That will change a lot,’ Doblin told PSYCH.

PTSD is a debilitating mental health condition triggered by witnessing or experiencing a traumatic event. Symptoms range from sleep disturbances and anxiety to paralysing flashbacks, causing the patient to feel as if they are reliving the event.
Study participants included patients with PTSD caused by combat-related events, accidents, abuse or sexual harm; 84% have a history of developmental trauma.
Jennifer Mitchell, Ph.D., lead author of the paper, calls attention to the results for those with the dissociative subtype of PTSD, with depression, or for those who reported a history of alcohol or substance use.
‘People with the most difficult-to-treat diagnoses, often considered intractable, respond just as well to this novel treatment as other study participants. In fact, participants diagnosed with the dissociative subtype of PTSD experienced a greater reduction in symptoms than those without the dissociative subtype,’ Mitchell said.

The group is currently working to enrol participants in a second Phase 3 clinical trial. The FDA has granted permission for 50 patients to receive the treatment prior to FDA approval.
MAPS has plans for additional studies, including the treatment of other mental health conditions and treatment protocols including group and couples therapy.
Nature Medicine is expected to publish the new peer-reviewed paper detailing the results of the current study later this month.
‘The more that we destigmatise these drugs, the more we make them available to people, the more that we have licensed legalisation, the more we train people and peer support, then the more we will affect mental health more broadly.’


