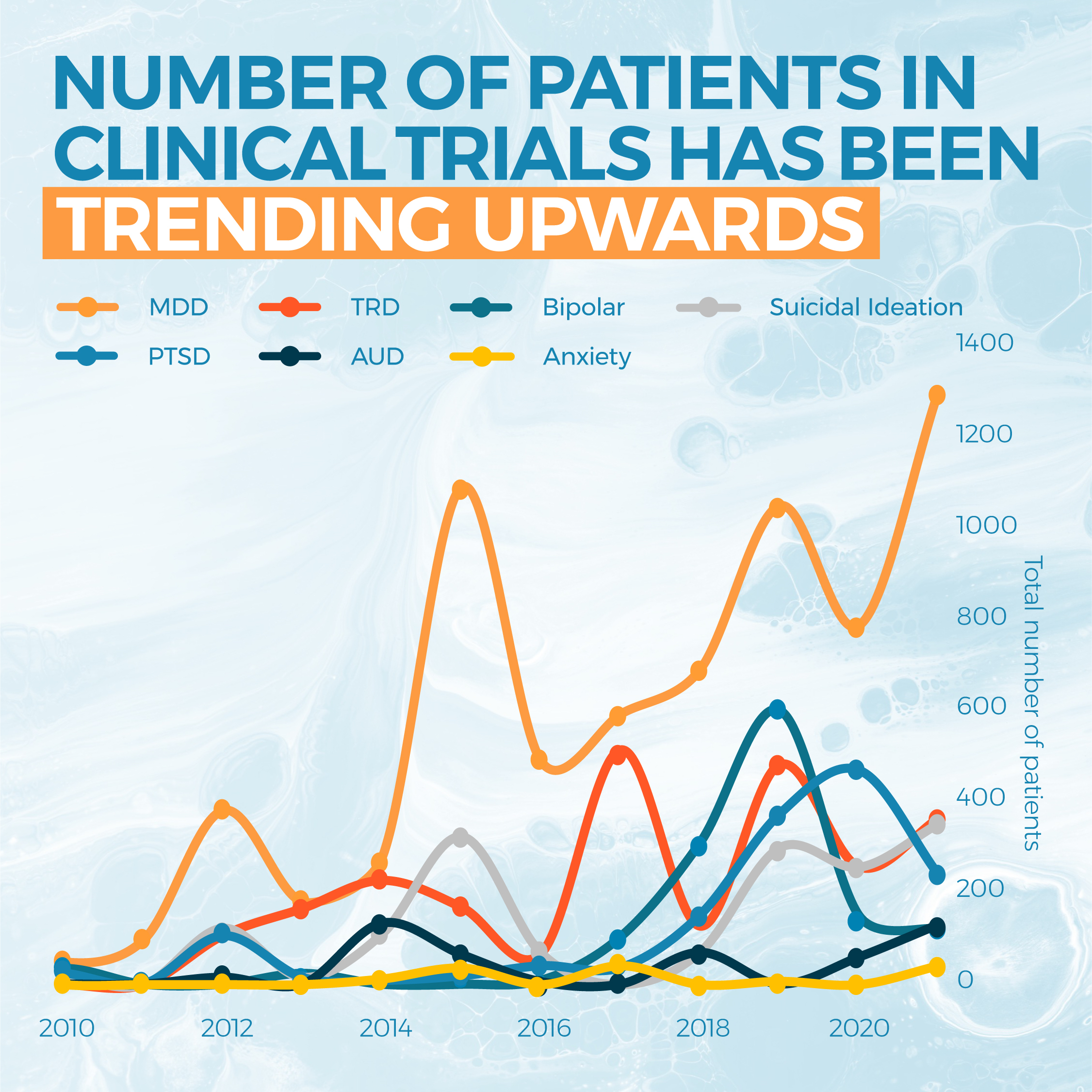
At the end of June, COMPASS Pathways announced it had administered its patented psilocybin-derivative, COMP360, to 216 patients in the largest psilocybin assisted-therapy trial to date. The Phase IIb clinical trial is examining the safety and efficacy of COMP360 to combat treatment-resistant depression, with the results expected to be published later this year.
Clinical trials are an essential building block in the development of psychedelic medicine, as the data informs the FDA, and other regulators, on the drug’s safety and suitability for medical application. As a result, drug developers rely on this scientific evidence to secure regulatory approval and pave the treatment’s route to market.
 On Wednesday, 7 July, McKee will be joined by Richard Carleton, CEO at Canadian Securities Exchange (CSE), Business Insider journalist Yeji Jesse Lee and Roger McIntyre, CEO at Braxia Scientific, to discuss the next decade of industry innovation. Early research indicates psychedelics could treat a plethora of physical and mental illnesses, but the market requires significant investment to realise its potential. The forward-looking panel discussion, The Next 5–10 Years of Research & Development, is one of several talks scheduled for the industry-leading global investor event, with the full agenda found here.
On Wednesday, 7 July, McKee will be joined by Richard Carleton, CEO at Canadian Securities Exchange (CSE), Business Insider journalist Yeji Jesse Lee and Roger McIntyre, CEO at Braxia Scientific, to discuss the next decade of industry innovation. Early research indicates psychedelics could treat a plethora of physical and mental illnesses, but the market requires significant investment to realise its potential. The forward-looking panel discussion, The Next 5–10 Years of Research & Development, is one of several talks scheduled for the industry-leading global investor event, with the full agenda found here.
After the panel session, McKee will deliver an investor-focused company presentation on Tryp’s successes to date and vision for the future. The address will discuss the company’s drug development opportunities and the benefits of engineering synthetic psychedelic compounds. The company presentation will be followed by a question-answer session, empowering investors to engage Tryp directly and explore commercial opportunities.
Common shares in Tryp are available on the OTCQB Venture Market under the symbol TRYPF, and TRYP on the Canadian Securities Exchange.
Have you registered to attend the PSYCH Investor Summit: Research & Development? If not, you can secure your ticket here.


