Psychedelic profiles: psilocybin
Psilocybin-assisted therapy for major depressive disorder and treatment-resistant depression are in Phase II trials, and may be approved by the FDA as early as 2025. The US federal department granted Breakthrough Therapy designation to the treatment, with 17 million people suffering from major depressive disorder in the US alone.
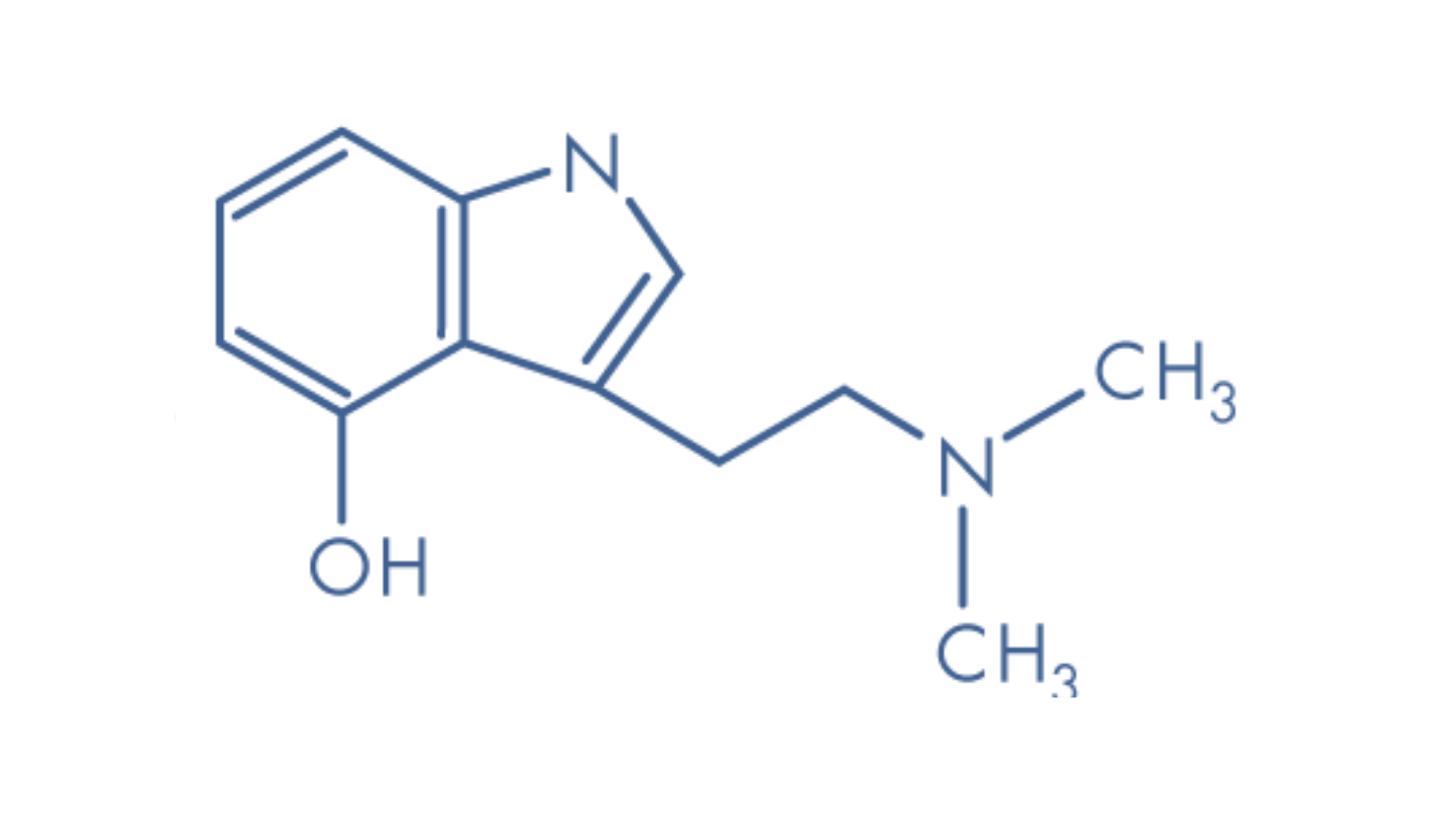
Psilocybin is a naturally occurring prodrug found in over 200 species of fungi. When ingested and metabolised, psilocybin converts into the tryptamine alkaloid psilocin, which imitates the neurotransmitter serotonin to produce psychoactive effects.
Brain imaging studies suggest high doses of psilocybin disrupt negative thought patterns through frenzied neurodynamics, which creates different neural pathways than those habitually used.
Read More