Cybin is conducting clinical trials with a number of tryptamines to treat a plethora of disorders. Today it was awarded
February 9, 2022
January 5, 2022
Last year MAPS completed its Phase IIIa trials with MDMA in the treatment of PTSD. The subsequent data release grabbed
December 29, 2021
2021 was a seminal year for psychedelic medicine, with data released from groundbreaking clinical trials harnessing MDMA and psilocybin. The
December 21, 2021
To alter public perception and promote the acceptance of psychedelics as medicines, Gwella launched Mojo – a functional mushroom product
December 15, 2021
For psychedelic medicines to reach as many patients as possible, developers need to secure buy-in at every level of the
December 8, 2021
Awakn Life Sciences recently signed a memorandum of understanding with Devon Partnership NHS Trust and the University of Exeter, to
December 1, 2021
In 2021, psychedelic medicines moved from the frontiers of scientific research to the front pages of newspapers worldwide. This was
November 23, 2021
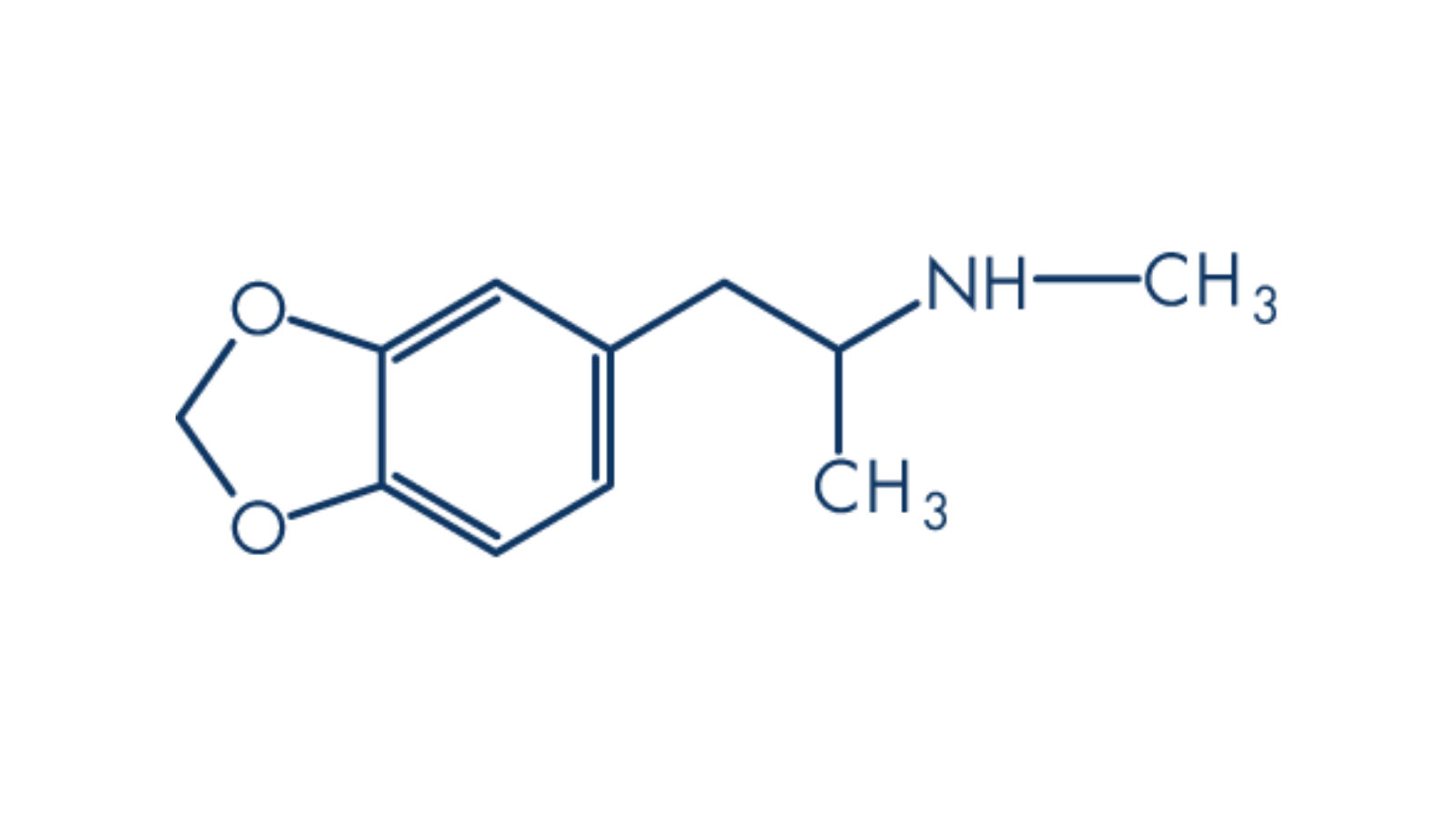
To strengthen the adoption of psychedelic-assisted therapies, drug developers are striving to minimise their cost – with shorter lasting tryptamines.
November 17, 2021
Member of Parliament Crispin Blunt has championed the campaign to reschedule psilocybin for scientific and research purposes in the UK.
November 10, 2021
This week COMPASS Pathways provided an eagerly anticipated update from its Phase IIb clinical trial. The largest ever study with
November 3, 2021
Cybin became the first psychedelic healthcare company to list on the New York Stock Exchange, with its psilocybin formulation to
October 27, 2021
At Prime Minister’s Questions on 19 October, Boris Johnson responded to Tory MP Crispin Blunt’s call to reschedule psilocybin. Johnson